The Life Cycle of Real World Data
To generate scientific evidence using RWD, Real World Evidence (RWE), FDA recommends establishing detailed, iterative, and ongoing processes to account for all of the information needed to review and reproduce all of the results.
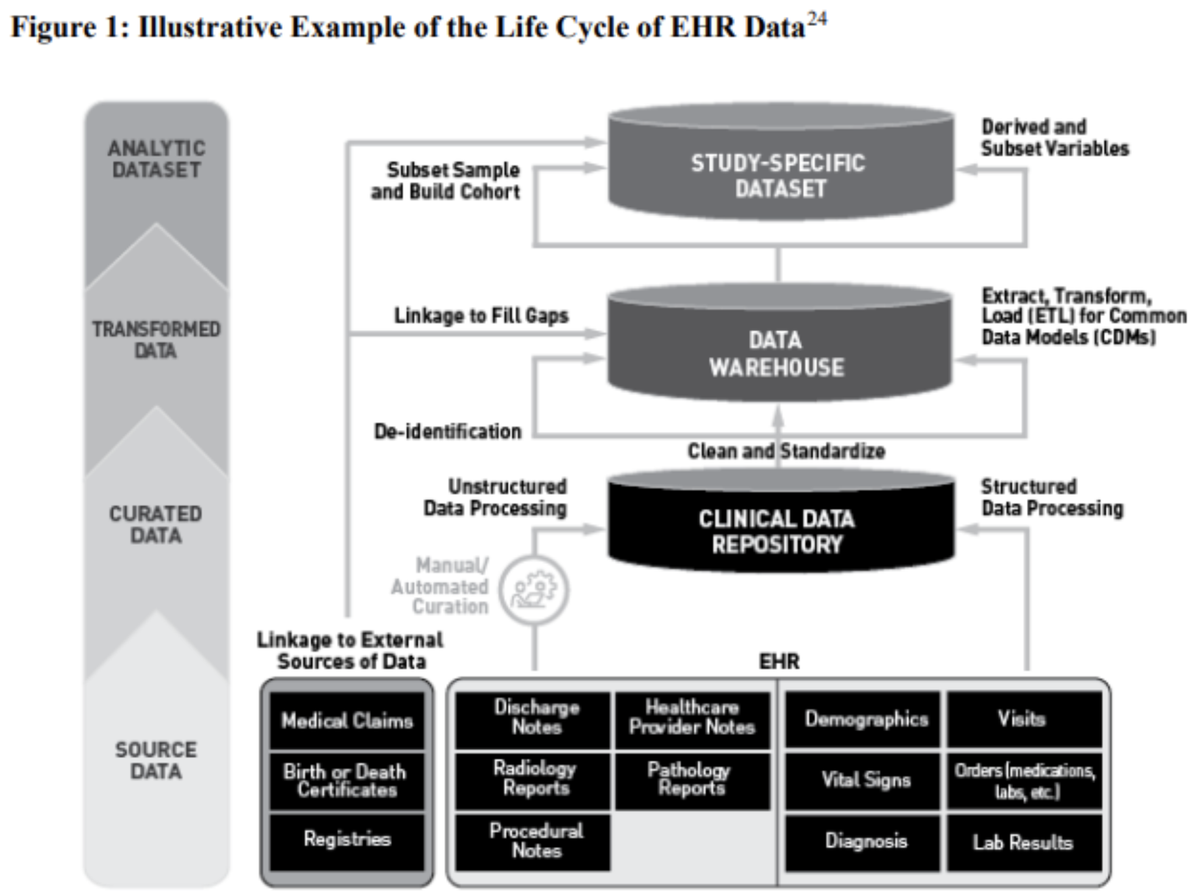
Figure 1 from the July 2024 FDA guidance entitled Assessing Electronic Health Records and Medical Claims Data To Support Regulatory Decision-Making illustrates the process of initial data capture to the derivation of a final analysis dataset by dividing it into 4 major phases: accrual, curation, transformation, and analysis. The process becomes a cycle as the study-specific analysis data accrues, potentially becoming the source of data for a subsequent study (e.g., longer and/or more widely-integrated).
When using EHR data sources, continuity of care should be discussed, specifically, whether patients receive all types of health care services within the same health system or network of facilities that contribute to the same EHR data source.
In general, the reliability of the data, as it culminates into the final analysis dataset, is assured by characterizing, and, where possible, quantifying, the accuracy and completeness of data across the life cycle while maintaining traceability throughout.
To satisfy these requirements, analysis documentation submitted to the FDA before initiating data analysis should specify the procedures by which traceability will be preserved. This includes describing the systems and processes that allow tracking the origin and transformations of data elements. At the time of submission, the supporting documentation (e.g., metadata, audit trails, coding system version histories, and access controls) enables the reconstruction of the data at each phase
FDA also recommends developing a Quality Assurance/Quality Control (QA/QC) plan to describe how sponsors will address issues and outline the tools and methods used to assess the data, generally requiring data quality checks, cleansing, and monitoring occur at every phase in the cycle.
References
- U.S. Food and Drug Administration (FDA). (2024, July). Assessing Electronic Health Records and Medical Claims Data To Support Regulatory Decision-Making. Retrieved from https://www.fda.gov/regulatory-information/search-fda-guidance-documents/real-world-data-assessing-electronic-health-records-and-medical-claims-data-support-regulatory > https://www.fda.gov/media/152503/download
